The SHINE trial, led by Dr Kate Hodgson and the Royal Women’s Hospital, is set to improve ‘intubation’ success and help newborn babies around the world.
Download the Australian Women's Weekly feature of this story – December 2022
Expert neonatologists and researchers at the Women’s have helped to establish a new method of care that could improve outcomes for newborns with breathing difficulties, and help train doctors globally.
More than 200 babies took part in the SHINE trial, which used heated, humidified oxygen to improve the safety and success of ‘intubation’, or breathing tube insertion.
‘High flow’ technology is considered non-invasive, and gently delivers a blend of air and oxygen through small prongs in the nose.
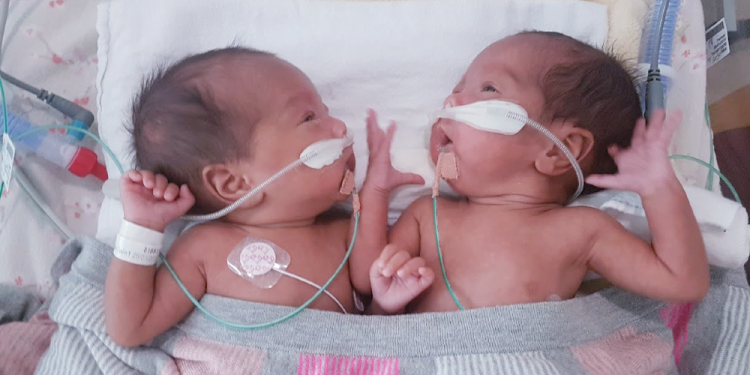
Ann Le gave birth to monochorionic-monoamniotic (MoMo) twins at just 28 weeks and five days at the Women’s in 2019. She enrolled her girls Zoe and Olivia in the SHINE trial, with both twins requiring breathing support.
“My girls are aged three now and completely healthy,” Ann said. “You wouldn’t even know they were 1KG each when born – and shared both an amniotic sac and a placenta.”
The twins stayed in the Women’s NICU for 11 weeks. They were reunited with their mum after an initial 27 days apart – hugging immediately and warming the hearts of Ann’s loved ones and Instagram followers.
Every year, more than 100 babies born over 12 weeks early and weighing less than 1KG are admitted to the Women’s NICU. Most will need a ventilator to support their breathing. For the SHINE trial, the average baby taking part was born at 28 weeks’ gestation.
The study showed that the use of ‘high flow’ increased the chance of a breathing tube being placed correctly on the first attempt, and that babies’ oxygen levels were more stable during the procedure. The technique is easy to apply and can be rolled out to hospitals everywhere.
“This widely available technology can help some of the most vulnerable babies we see born at the Women’s, or any hospital around the world,” said Dr Kate Hodgson, Neonatologist and lead researcher.
“This is the first trial around the world to look at this therapy in newborn babies. It is a simple method, which has a lot of potential. This innovation could help babies, both preterm and term babies, who are very unwell and need support with their breathing after birth.”
The study also found that the greatest benefits were seen when less experienced clinicians were performing the intubation.
Ann, who is also a clinic co-ordinator at the Royal Children’s Hospital, encourages any parents asked to enrol their newborns in research to take up the offer.
“I am so grateful that Olivia and Zoe received such a high standard of care at the Royal Women’s Hospital, and were also able to contribute to something as important as the SHINE trial,” she said.
“If my girls were born in the 90s I’m not sure they would have made it. Research in this area is what helps saves lives now, and well into the future.
“Any medical procedure, especially on a baby, is so scary for parents. If you can get that done quickly and have better outcomes – that would be so valuable to parents all over the world.”
|
Notes on the SHINE trial
|
.png)